このアプリのYoutube動画がある場合はURLを送信してください。詳細 »
APPLICATION INFO - iPhoneアプリの詳細情報
![]()
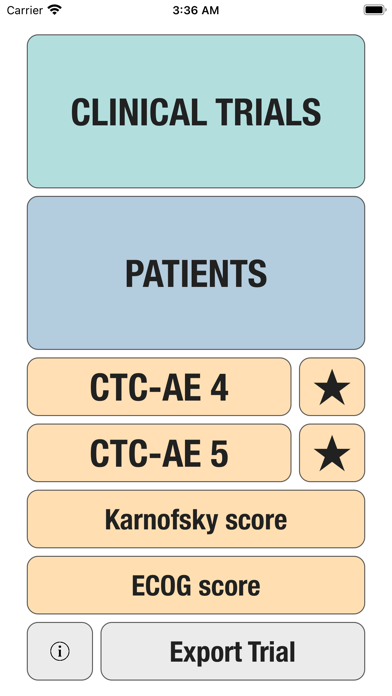
CTC-AE+ is a browsable reference to the CTC-AE list of adverse event (AE) terms commonly encountered in oncology, plus a portable Adverse Event Logger to keep track of all adverse events during a clinical study.
The CTC-AE 4 and CTC-AE 5 have been developed from the earlier vocabulary known as CTC (Common Toxicity Criteria). Each AE term is defined and accompanied by a grading scale that indicates the severity of the adverse event. All AE terms are organized by the System Organ Classes (SOCs) defined by the Medical Dictionary for Regulatory Activities (MedDRA).
Adverse events are common phenomena affecting patients being treated for cancer. With the availability of new agents and the multimodality interventions, it is critical to monitor systematically the AEs that are linked to oncology research.
CTCAE is fundamentally intended to be an agreed upon terminology for the designation, reporting, and grading of AEs that occur in oncology research.
########################
CTC-AE serves several purposes
########################
- To standardize AE reporting within the NCI oncology research community, across groups and modalities.
- To facilitate the evaluation of new cancer therapies, treatment modalities, and supportive measures.
- To aid in AE recognition and severity grading.
- To monitor safety data and for regulatory reporting.
- To define oncology research protocol parameters (e.g., eligibility criteria; dose-limiting toxicity; maximum tolerated dose; dose modification).
########################
KARNOFSKY and ECOG
########################
Additionally, CTC-AE+ now includes the two most common performance scores. As a reference and as a guided algorithm when necessary to add the event to the patient's record.
########################
ADVERSE EVENT LOGGER
########################
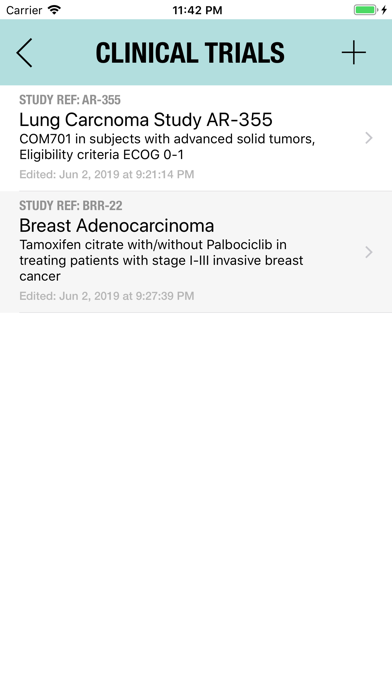
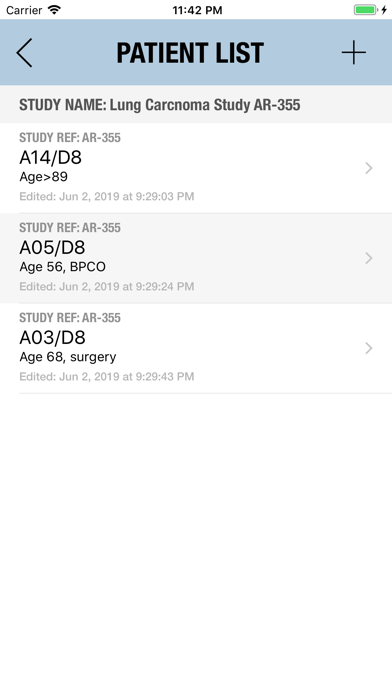
The Adverse Event Logger is a structured local database to help the investigator to keep track of all the adverse events related to the patients participating in a clinical trial.
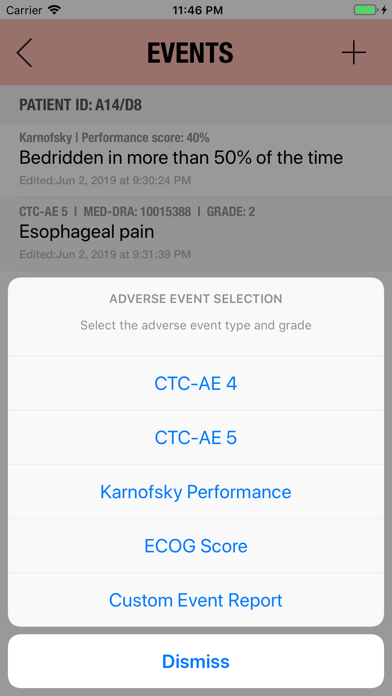
It is possible to track multiple clinical studies, multiple patients per clinical trial and multiple events per patient. Events can be selected from the CTC-AE 4 and CTC-AE 5 databases, from Karnofsky and ECOG score tables or can be entered as customized events.
For convenience, all data can be exported as an excel CSV file to be imported into other analysis software.
########################
IMPORTANT NOTE
########################
This application is strictly EU GDPR compliant and no clinical study or patient information is saved outside the device or uploaded to any remote cloud resources.
The CTC-AE 4 and CTC-AE 5 have been developed from the earlier vocabulary known as CTC (Common Toxicity Criteria). Each AE term is defined and accompanied by a grading scale that indicates the severity of the adverse event. All AE terms are organized by the System Organ Classes (SOCs) defined by the Medical Dictionary for Regulatory Activities (MedDRA).
Adverse events are common phenomena affecting patients being treated for cancer. With the availability of new agents and the multimodality interventions, it is critical to monitor systematically the AEs that are linked to oncology research.
CTCAE is fundamentally intended to be an agreed upon terminology for the designation, reporting, and grading of AEs that occur in oncology research.
########################
CTC-AE serves several purposes
########################
- To standardize AE reporting within the NCI oncology research community, across groups and modalities.
- To facilitate the evaluation of new cancer therapies, treatment modalities, and supportive measures.
- To aid in AE recognition and severity grading.
- To monitor safety data and for regulatory reporting.
- To define oncology research protocol parameters (e.g., eligibility criteria; dose-limiting toxicity; maximum tolerated dose; dose modification).
########################
KARNOFSKY and ECOG
########################
Additionally, CTC-AE+ now includes the two most common performance scores. As a reference and as a guided algorithm when necessary to add the event to the patient's record.
########################
ADVERSE EVENT LOGGER
########################
The Adverse Event Logger is a structured local database to help the investigator to keep track of all the adverse events related to the patients participating in a clinical trial.
It is possible to track multiple clinical studies, multiple patients per clinical trial and multiple events per patient. Events can be selected from the CTC-AE 4 and CTC-AE 5 databases, from Karnofsky and ECOG score tables or can be entered as customized events.
For convenience, all data can be exported as an excel CSV file to be imported into other analysis software.
########################
IMPORTANT NOTE
########################
This application is strictly EU GDPR compliant and no clinical study or patient information is saved outside the device or uploaded to any remote cloud resources.
 このアプリはiPhone、iPadの両方に対応しています。
このアプリはiPhone、iPadの両方に対応しています。
カテゴリー
メディカル
メディカル
リリース
2010/1/7
2010/1/7
更新
2021/5/17
2021/5/17
バージョン
3.1
3.1
言語
英語
英語
サイズ
14.2 MB
14.2 MB
条件
iPhone、iPod touch および iPad 互換 iOS 3.0 以降が必要
iPhone、iPod touch および iPad 互換 iOS 3.0 以降が必要
このバージョンの新機能
Added CTC-4 and CTC-5 bookmarks for the most common adverse events.
Added CTC-4 and CTC-5 bookmarks for the most common adverse events.
スクリーンショット - iPhone | iPad
スクリーンショット - iPhone | iPad
Arpacore B.V. の他のアプリ » もっと見る
» 目的別iPhoneアプリ検索
- 辞書 » 日本語対応の辞書 » 英和辞典
- 学習 » 英単語 » 英会話 » クイズで楽しく
- スケジュール » 管理 » Googleカレンダー





たまひよ 妊娠・育児の赤ちゃんとママパパ応援アプリ まいにち..
Benesse Corporation無料

無料 赤ちゃん名づけ 400万人の妊婦さんが子供の名付けで利..
Recstu Inc.無料

第76回日本臨床外科学会総会 Mobile Planner
Japan Convention Services, Inc..無料

陣痛時計
Simple Beep無料

Yahoo!家庭の医学 - 症状や治療法を解説
Yahoo Japan Corp.無料

たまひよ妊娠カウンター【たまプレ】
Benesse Corporation無料

ナスカレ≪看護師のシフト表≫ナースのシフト共有カレンダーアプ..
株式会社クイック無料

ストレススキャン カメラで手軽にストレスチェック!
Stress Scan Inc.無料

赤ちゃん泣き止み音アプリ
BookLive Co., Ltd.無料

きほんの離乳食 管理栄養士が監修したレシピが300種類以上!
Palsystem Consumers' Co-operat..無料
CatchApp新着アプリレビュー

様々な楽しみ方で運転士として成長していく鉄道運転士育成RPG「プラチナ・トレイン(プラトレ) 日本縦断てつどうの旅」
2016-05-17 00:00

日本語にはない英語発音のリスニングを楽しく学べる「発音どっち?英語リスニング 」
2014-12-20 12:00

指先の瞬発力が試されるカジュアルゲーム「早撃ちパニック」をリリース!
2014-12-08 15:21
新着アプリ動画